Abstract
Background Relapsed or refractory (R/R) transformed indolent B-cell non-Hodgkin lymphoma (iB-NHL) is associated with a poor prognosis. Unfortunately, there is little prospective data using novel agents, including kinase inhibitors, since trials tend to specifically exclude transformed disease. To address this question we evaluated single agent ibrutinib in patients with R/R transformed iB-NHL.
Methods This was a single-arm, open-label phase II trial for patients with R/R transformed iB-NHL (NCT02207062). Eligibility included no prior treatment with ibrutinib, ECOG 0-2, measurable biopsy-proven transformed iB-NHL, and prior receipt of at least one line of therapy for the transformed disease. Treatment consisted of oral ibrutinib 560 mg daily until disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed overall response rate (ORR, per Lugano criteria), with a predefined threshold of success ≥ 30%. Secondary endpoints included clinical benefit rate (CBR, defined as ORR and/or stable disease of ≥ 6 months), progression-free survival (PFS), and safety.
Results As of July 1, 2018, 17 patients were treated. The median age was 68 years (range, 36 to 89) and 53% were women. Median number of treatment regimens prior to enrollment was 4 (range 2-9) and 41% had no response to their last prior treatment. Prior therapies included rituximab (100%), anthracycline (100%), platinum (76%), and a novel agent on protocol (36%). Prior autologous hematopoietic stem cell transplantation (HSCT) had been performed in 47% and prior CAR-T in 12%. The most common histologies of the indolent B-NHL included follicular lymphoma (65%), small lymphocytic lymphoma (12%), and lymphoplasmacytic lymphoma (12%). Histology of the transformed disease was diffuse large B cell lymphoma in all cases, and included germinal center B-cell (GCB) subtype by immunohistochemistry in 71% and MYC rearrangement in 12%. Five patients had bulky disease (at least one tumor mass > 5 cm in largest dimension) at the start of treatment.
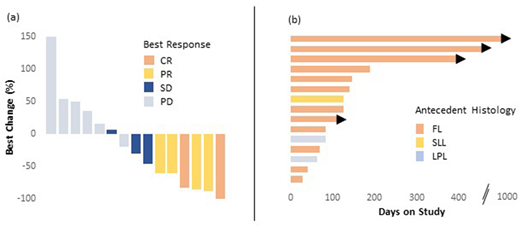
Fifteen patients were evaluable for response at the time of this data cut (Figure 1). The ORR was 40% and CBR was 47% with complete response (CR) seen in 2 patients (13%). Median PFS was 4.1 months and median duration of response was 6.8 months; estimated 12-month PFS was 23%. The 2 patients that achieved CR continue on study in remission at 37 and 15 months, respectively. ORR was associated with disease bulk, with 60% ORR in non-bulky vs 0% ORR in cases of bulky disease (p = 0.04). ORR was not associated with cell of origin (42% in GCB subtype vs 33%) or MYC rearrangement (50% in MYC rearrangement vs 35%). After treatment with ibrutinib, 2 patients proceeded to CAR-T and 1 of these subsequently underwent allogeneic HSCT. No deaths or unexpected toxicities occurred with these therapies.
The side effect profile was consistent with other trials with most common adverse events (AEs) for all pts being grade 1-2 and including fatigue (59%), bruising (53%), and diarrhea (24%). Grade ≥ 3 AEs occurred in 8 patients (47%) including neutropenia (6%), lymphopenia (6%), esophagitis (6%), nausea (6%), pneumonia (6%), sepsis (6%), and atrial flutter (6%). Treatment was discontinued due to AE (grade 3 mucositis) in 1 patient after 3 weeks on therapy. One patient died while on treatment due to unknown cause.
Conclusions Ibrutinib appears active in patients with R/R transformed iB-NHL that had previously received multiple agents, including rituximab and anthracycline therapy in all cases. Prolonged PFS was observed in some patients, with an estimated 12-month PFS of 23%, with higher efficacy seen in those with lower disease burden. These pilot data indicate that trials in patients with R/R transformed lymphoma can be accomplished and that ibrutinib may be clinically beneficial to a subset of such patients. The final outcome data for full accrual (n = 20) will be reported in December.
Figure 1 Waterfall (a) and swimmers (b) plots. In 1a, the best response is noted: CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease. In 1b, the antecedent indolent histology is noted: FL = follicular lymphoma; LPL = lymphoplasmacytic lymphoma; SLL = small lymphocytic lymphoma. Arrowhead = continues on treatment.
Graf:Beigene: Research Funding; TG Therapeutics: Research Funding; Acerta: Research Funding. Cassaday:Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Seattle Genetics: Other: Spouse Employment, Research Funding; Kite Pharma: Research Funding; Incyte: Research Funding; Jazz Pharmaceuticals: Consultancy; Merck: Research Funding; Adaptive Biotechnologies: Consultancy. Becker:GlycoMimetics: Research Funding. Smith:Incyte Corporation: Research Funding; Portola Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Merck Sharp and Dohme Corp.: Consultancy, Research Funding; Acerta Pharma BV: Research Funding; Genentech: Research Funding; Seattle Genetics: Research Funding. Gopal:Incyte: Consultancy; Teva: Research Funding; Spectrum: Research Funding; Brim: Consultancy; Pfizer: Research Funding; Merck: Research Funding; Aptevo: Consultancy; Asana: Consultancy; Takeda: Research Funding; Seattle Genetics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal